Abstract
Introduction: the effect of rituximab on response rates and survival in Primary Central Nervous System Lymphoma (PCNSL) has not been studied in a phase III randomized clinical trial. Its use in this setting is based on extrapolations of its major impact on survival rates in the systemic B cell lymphoma setting, and on phase II and retrospective PCNSL studies. A major concern is whether the low penetration of the large rituximab molecule through the blood brain barrier can result in therapeutic concentrations within the brain parenchyma. We performed an international intergroup randomized phase III study to investigate the efficacy of rituximab in PCNSL.
Methods: newly-diagnosed, non-immunocompromized patients (aged 18-70 years) with PCNSL and ECOG performance status (PS) 0-3 were randomized to induction with 2 cycles of MBVP (high dose methotrexate, BCNU, teniposide, prednisone) chemotherapy with (arm B) or without (arm A) rituximab. Patients were stratified for age (≤60 vs ≥61 years) and PS (0-1 vs 2-3). The rituximab (375 mg/m2) was given weekly during MBVP cycle I (on day 0 and 14) and every other week during cycle II to a total of 6 administrations. Patients with persistently positive CSF after the first (R-)MBVP cycle received intrathecal MTX. Patients with at least a partial response received consolidation with high dose cytarabine. Subsequently, patients ≤ 60 years were treated with 30 Gy whole brain radiotherapy (WBRT) with an additional boost of 10 Gy in case of partial remission. Patients ≥61 yrs were not to be irradiated. MRI evaluation was planned after 2 cycles of (R)-MBVP, after high dose cytarabine and, in case of WBRT, 4 weeks after the WBRT. In follow-up MRI was scheduled every 3 months for the first two years, every six months in years 3-5, and annually thereafter. Primary end-point was event free survival (EFS), where an event was no CR(u) on protocol, relapse or death. Secondary end-points were response rates after induction, consolidation and completion of radiotherapy, as well as progression free and overall survival.
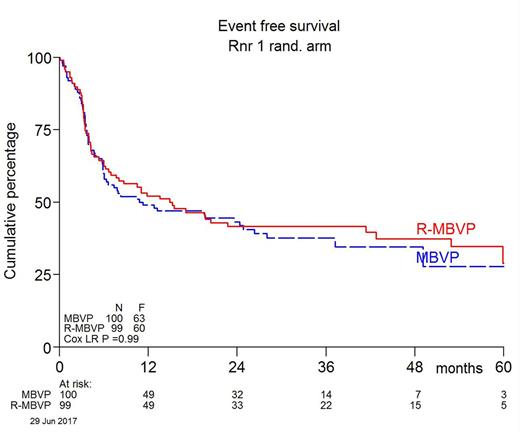
Results: between August 2010 and June 2016, 200 patients from 24 centers in the Netherlands, Australia and New Zealand were included and randomized; 100 patients were randomized to arm A and 99 to arm B, 1 was not eligible and excluded from all analyses. 55% were men. The median age was 61 years (range 26-70), 47% were ≤60 years, 72% had an ECOG PS ≤ 1, 29% had elevated LDH and 30% had positive CSF; the two arms were well balanced at baseline. All patients received cycle I according to randomization, 90% received cycle II and 81% received high dose cytarabine. 16 patients, 8 in each arm, received intrathecal treatment. 70 patients (35%) were irradiated, 34% in arm A and 36% in arm B. A boost was given to 15/34 patients in arm A (44%) and 24/36 patients in arm B (67%). CR/CRu rate was 66% in arm A and 68% in arm B. Overall response rate was 87% in both arms. EFS did not differ between the arms: 1 year EFS was 49% in arm A and 52% in arm B; HR 1.00, 95% CI 0.70-1.43, p=0.99 adjusted for age group and WHO. An exploratory multivariate analysis revealed a significant age-arm interaction effect suggesting a possible effect of rituximab in younger patients; within this subgroup, HR = 0.54, 95% CI 0.30-0.98. HR for PFS adjusted for age and WHO was 0.77 (95% CI 0.52-1.13, p=0.18). OS data are not yet mature as median OS has not been reached after a median follow-up of 32.9 months (range 3.6-79.2) in the 120 patients still alive. However, no difference was apparent with an adjusted analysis HR of 0.93 (95% CI 0.59 - 1.44, p=0.74). At database lock 79 patients had died; treatment related mortality was 7% in arm A and 3% in arm B. Grade 3 or 4 adverse events occurred in 59% of patients in arm A, in 63% in arm B, of which infections were the most frequent, occurring in 23% and 21% of patients respectively.
Conclusions: in this randomized phase III trial in newly diagnosed PCNSL, the addition of rituximab to HD-MTX-based chemotherapy did not improve response rates, EFS or PFS. Longer follow up is needed to evaluate the effect on overall survival.
Minnema: Janssen: Consultancy; Celgene: Consultancy, Research Funding; Takeda: Consultancy; Amgen: Consultancy; Servier: Consultancy. Cull: Amgen Australia: Other: travel expenses Lugano lymphoma conference 2017; Takeda Australia: Other: travel expenses Highlights of ASH march 2017, Brisbane. Nijland: Novartis Pharmaeuticals Corporation: Honoraria; Kite Pharma: Honoraria; Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; MIllennium/Takeda: Honoraria, Research Funding; Mundipharma: Honoraria; Gilead Sciences: Honoraria; BMS: Honoraria; MSD: Honoraria; Amgen: Honoraria. van den Bent: Roche: Consultancy. Jong: BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy; Celgene: Consultancy, Research Funding; Genmab: Research Funding. Doorduijn: Celgene: Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal